In Depth Look at Extractive Distillation
- Distillation for azeotropic mixture
Distillation is the most widely used separation technique
in the chemical and petroleum industry. However, not all liquid mixture are
amenable to ordinary
fractional distillation. When the components of the system have low relative
volatilities (1.00 < a < 1.05), separation
becomes difficult and expansive because a large number of trays are required
and, usually, a high reflux ratio as well. Both equipment and utilities
costs increase markedly and the operation can become uneconomical. If the
system forms azeotropes, as in a benzene and cyclohexane system, a different
problem arises, - the azeotropic composition limit the separation, and for a
better separation this azeotrope must be bypassed in some way. At low to
moderate pressure, with the assumption of ideal-gas model for the vapor
phase, the vapor-liquid phase equilibrium (VLE) of many mixture can be
adequately describe by the following Modified Raoult�s Law:
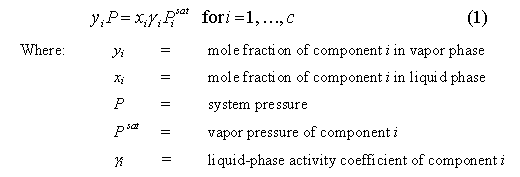
When gi = 1,
the mixture is said to be ideal Equation 1 simplifies to Raoult�s Law.
Nonideal mixtures (gi
� 1) can exhibit either positive (gi
> 1) or negative deviations (gi
< 1) from Raoult�s Law. In many highly nonideal mixtures these
deviations become so large that the pressure-composition (P-x, y) and
temperature-composition (T-x, y) phase diagrams exhibit a minimum or
maximum azeotrope point. In the context of the T-x, y phase diagram,
these points are called the minimum boiling azeotrope (where the
boiling temperature of the azeotrope is less than that of the pure
component) or maximum boiling azeotrope (the boiling temperature of
the azeotrope is higher than that of the pure components). About 90% of the
known azeotropes are of the minimum variety. At these minimum and maximum
boiling azeotrope, the liquid phase and its equilibrium vapor phase have the
same composition, i.e.,
xi = yi for i = 1, �, c (2)
Two main types of azeotropes exist, i.e. the
homogeneous azeotrope, where a single liquid phase is in the equilibrium
with a vapor phase; and the heterogeneous azeotropes, where the
overall liquid composition which form two liquid phases, is identical to the
vapor composition.
Most methods of distilling azeotropes and low relative
volatility mixtures rely on the addition of specially chosen chemicals to
facilitate the separation. The selection of the separating agent will be
discussed later.
The five methods for separating azeotropic mixtures are:
- Extractive distillation and homogeneous azeotropic
distillation where the liquid separating agent is completely
miscible.
- Heterogeneous azeotropic distillation, or more commonly,
azeotropic distillation where the liquid separating agent,
called the entrainer, forms one or more azeotropes with the other
components in the mixture and causes two liquid phases to exist over
a wide range of compositions. This immiscibility is the key to
making the distillation sequence work.
- Distillation using ionic salts. The salts dissociates in
the liquid mixture and alters the relative volatilities sufficiently
that the separation become possible.
- Pressure-swing distillation where a series of column
operating at different pressures are used to separate binary
azeotropes which change appreciably in composition over a moderate
pressure range or where a separating agent which forms a
pressure-sensitive azeotrope is added to separate a
pressure-insensitive azeotrope.
- Reactive distillation where the separating agent reacts
preferentially and reversibly with one of the azeotropic
constitutes. The reaction product is then distilled from the
nonreacting components and the reaction is reversed to recover the
initial component.
|

